Thermite Grenades are fun. If you’ve ever gotten to use one to slag anything, you know. They make joyous melted mess of just about anything (we did a stack of empty ammo cans) from engines to electronics… anything vulnerable to molten metal being poured into it really.
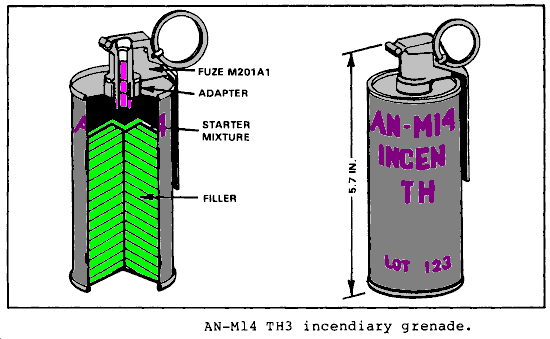
Today’s Slo-Mo guys video goes into the thermite reaction and a little about why normal fire fighting techniques do nothing. Essentially it’s because this isn’t fire. Fire uses environmental oxygen where thermite does not, it’s reaction is self sustaining. Water doesn’t deprive the thermite of nearly enough heat to stop the reaction and since the reaction does not use oxygen from the atmosphere water doesn’t deprive it of that either.
Advertisement — Continue Reading Below
The most common form of thermite, although many metallic/oxide combinations can produce this high temperature reaction, is a combination of rust (iron oxide) and aluminum. Aluminium is preferred because the reaction quickly hits its melting point temperature without going over its boiling point, so the whole shiny hot display stays as molten liquid metal instead of exploding into gas. Now it will obviously flash boil items like water (you can see it in the video) that it comes into contact with and anything that has an ignition temperature the mixture exceeds is going to burn.
Result, one can of spicy shiny melty goodness that can be transported very safely and stably. It needs a high temperature to set it off so a high temperature fuse is used in the grenades and things like magnesium cord are used in other applications. Applications like slow motion video.
Enjoy!
Advertisement — Continue Reading Below















